Some hypotheses about a possible connection between malaria and jet-lag
Malaria Action Day is today, March 19th. Read Tara, Tim and me for more information. You should play basketball today, as part of the Dunk Malaria initiative. You should donate to The Global Fund for the fight against malaria (and send Tim the confirmation e-mail you get, so he can match it up to $300). Post this information on your blog (or e-mail to friends) today. Write a blog-post or journal entry about malaria or something related and I will put together a linkfest on Sunday (on Science And Politics), linking to all bloggers who send me permalinks of their posts on the topic. If you have not written anything recently, but have a good post from the past, send it anyway. The posts need not focus on biology or medicine of malaria - writings on history, geography, economics and politics of this disease are equally welcome.
I have written a little bit about malaria before, e.g, here and here, but this is my special Malaria Action Day post, inspired by a paper [1] that Tara sent me some weeks ago and I never got to write about it till now.
-------------------------------------------------------------------------------
In a journal called "Medical Hypotheses" Kumar and Sharma [1] propose that jet-lagged travellers may be more susceptible to getting infected with malaria. They write:
1) affect the rate/ease of infection with malaria,
2) affect the symptoms of malaria in an infected individual,
3) affect the ability of the body to fight off the infection,
4) affect the effectiveness of treatments, and
5) affect the likelihood that the infected individual will spread the disease to others.
The Kumar/Sharma hypothesis is clearly of the #1 type. I will look more at other types of hypotheses - those that apply to already infected individuals. For that, let's first go quickly through the basic biology of malaria.
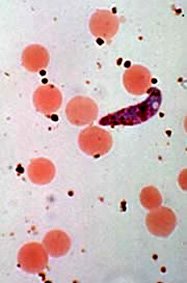 Malaria is caused by a protist in the genus Plasmodium. While Plasmodium falciparum is the most common species, three or four other species are also causes of malaria in humans, and dozens of other species cause malaria or malaria-like diseases in other animals, including mammals, birds and reptiles.
Malaria is caused by a protist in the genus Plasmodium. While Plasmodium falciparum is the most common species, three or four other species are also causes of malaria in humans, and dozens of other species cause malaria or malaria-like diseases in other animals, including mammals, birds and reptiles.
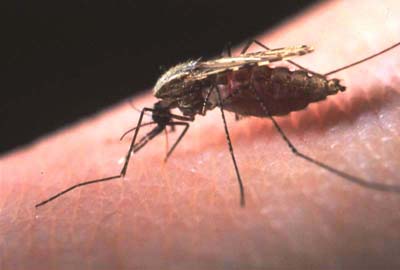 Plasmodium is transmitted through bites of several species of mosquito from the genus Anopheles. Once injected into the final host (e.g., human), the plasmodia remain in the skin for several hours, then migrate to lymph nodes, spleen and liver where they undergo several transformations. The final stage - the gametocyte - migrates into the red blood cells. Inside each red blood cell one can find a large number of plasmodia, hiding there from the immune system of the host. The whole life-cycle lasts several days, even weeks to complete.
Plasmodium is transmitted through bites of several species of mosquito from the genus Anopheles. Once injected into the final host (e.g., human), the plasmodia remain in the skin for several hours, then migrate to lymph nodes, spleen and liver where they undergo several transformations. The final stage - the gametocyte - migrates into the red blood cells. Inside each red blood cell one can find a large number of plasmodia, hiding there from the immune system of the host. The whole life-cycle lasts several days, even weeks to complete.

All the plasmodia burst out of red blood cells simultaneously. Enormous number of plasmodia suddenly released into the blood overwhelms the immune system of the host, allowing the plasmodia to survive unscathed for quite a long time. This time is sufficient for them to invade blood vessels in the skin where, if they are lucky, a mosquito will bite and the plasmodia can invade the mosquito again and search for the next host.
 The bursting of red blood cells triggers high fever and sweating. High temperature, high carbon-dioxide, as well as some odors [2] present in the sweat are all highly attractive to mosquitoes, rasing the probability that the host will get bitten. In some species of Plasmodium (like P.falciparum), the bursting of red blood cells occurs every night. In some species of Plasmodium, the resulting fever occurs every two nights and in some every four nights (rarely three), causing, respectively, tertian and quartan fevers. Tertian and quartan malaria are treated by chloroquine, while falciparum malaria is treated by quinine, mefloquine or halofantrine.
The bursting of red blood cells triggers high fever and sweating. High temperature, high carbon-dioxide, as well as some odors [2] present in the sweat are all highly attractive to mosquitoes, rasing the probability that the host will get bitten. In some species of Plasmodium (like P.falciparum), the bursting of red blood cells occurs every night. In some species of Plasmodium, the resulting fever occurs every two nights and in some every four nights (rarely three), causing, respectively, tertian and quartan fevers. Tertian and quartan malaria are treated by chloroquine, while falciparum malaria is treated by quinine, mefloquine or halofantrine.
Obviously, from the perspective of a Plasmodium, timing is crucial. First, it is important to errupt in synchrony. Yet, hidden inside red blood cells, plasmodia cannot communicate with each other. Second, it is important to time the eruption in such a way as to maximize the probabilty that some of the gametocytes will be picked up by mosquitoes. Thus, it is important for the eruption to occur at the time of day when mosquitoes are most actively foraging for blood.
How do the Plasmodia solve the problem of timing? This is where circadian biology comes in [3,4,5]. Plasmodia residing inside red blood cells use the time-clues generated by the host. More specifically, they key onto the nightly release of melatonin by the pineal gland. Melatonin is practically undetectable in the blood during the day and the concentrations rise steeply in the evening remaining high for the duration of the night (exact patterns differ between vertebrate species), then dropping again at dawn.
Plasmodia have melatonin receptors [3]. Interestingly, unlike melatonin receptors in vertebrates which are nuclear receptors, the receptors in Plasmodia are membrane receptors. Membrane receptors are much faster than nuclear receptors which is important when a biological event has to be timed with precision. However, the plasmodia do not destroy the red blood cell immediately after receiving the melatonin signal - that would be too early in the evening for the timing to be adaptive, as the mosquitoes are still too busy looking for mates and mating at that time. Instead, the plasmodia use their own circadian clocks to measure the exact timing of eruption. In a way, it appears that the host melatonin signal entrains (synchronizes) the clocks in plasmodia, and then the Plasmodium clock determines the phase (exact timing) for the eruption out of red blood cells.
Different species of Anopheles and even geographically distinct populations of the same species have different times of peak foraging (biting) activity. In each geographical region, the local population (or species) of Plasmodium evolved the timing of eruption to match that of the local mosquitoes.
Let's now introduce another player. Apart from the parasite (Plasmodium), the host (a vertebrate, e.g., a human), and the vector (mosquito), one should also consider the predator - insectivorous bats that hunt for mosquitoes. The way that the malaria literature tends to think about timing can schematically be presented like this:
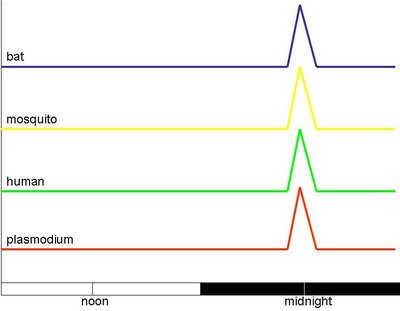 There is an assumption that plasmodium eruption, human fever, mosquito foraging and bat hunting are all synchronous. We have already looked at this from the perspective of the Plasmodium - it is adaptive for the Plasmodium for the three bottom lines to be accurate, i.e, that the parasite, the host and the vector are in synchrony. This also means that this is maladaptive to humans. It is also maladaptive to mosquitoes whose fitness does suffer somewhat if they are loaded with parasites.
There is an assumption that plasmodium eruption, human fever, mosquito foraging and bat hunting are all synchronous. We have already looked at this from the perspective of the Plasmodium - it is adaptive for the Plasmodium for the three bottom lines to be accurate, i.e, that the parasite, the host and the vector are in synchrony. This also means that this is maladaptive to humans. It is also maladaptive to mosquitoes whose fitness does suffer somewhat if they are loaded with parasites.
On the other hand, it is maladaptive for mosquitoes and plasmodia, and adaptive for humans and bats, if the peak hunting time for bats coincides with the peak foraging time of mosquitoes. More these two events are in sync, more mosquitoes will get eaten, thus less plasmodia will get into a new host and less humans will get infected.
The dynamics of the timing relationship between the four species can be described as an Evolutionary Arms-Race Around The Circadian Clock. While some of the players will try to maximize their fitness by achieving synchrony, the other players maximize their fitness by avoiding synchrony with each other. This can be depicted, for bats and mosquitoes, like this:
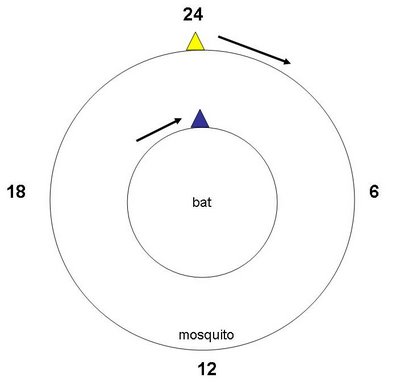 In this case, mosquitoes evolve to forage at later times of night, and bats evolve to track the mosquitoes by hunting later at night. This can go on back and forth endlessly. But, and here is a big "but". This model is quite oversimplified as it posits only four players and for each player an absolute loyalty to the other three. But is the real world that simple?
In this case, mosquitoes evolve to forage at later times of night, and bats evolve to track the mosquitoes by hunting later at night. This can go on back and forth endlessly. But, and here is a big "but". This model is quite oversimplified as it posits only four players and for each player an absolute loyalty to the other three. But is the real world that simple?
Plasmodium species are pretty host-specific. Species that thrive inside humans, may not thrive or even survive inside the bodies of other animals and vice versa. So, the parasite is pretty loyal to its host. It is also completely dependent on Anopheles - it will most likely not survive inside a different kind of mosquito.
The same mosquito that usually bites a human will happily take a blood meal from another animal. This is actually used as one of the prevention techniques: a village is surrounded by fields full of cattle, sheep, goats, horses, donkeys or camels. The mosquitoes coming out of the woods at night encounter these animals first and get satiated with blood before they ever encounter humans. The animals themselves do not get sick.
Bats are unlikely, in my opinion, to be specialized on Anopheles as their only prey. If there are no mosquitoes around, they will happily hunt other insects (and the tropical regions where malaria is common are swarming with many species of insects!). I think that involvement of bats in the arms-race is the weakest aspect of the hypothesis. Here are four basic types of bat hunting activity that are theoretically possible:
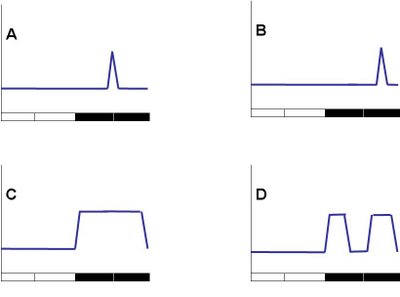 The hypothesis suggests that bats mostly fly around midnight when the mosquitoes are most active, i.e., the bats are winners and mosquitoes loosers in the arms-race (A) . If the peak is at some other point during the night, that would suggest that bats are involved in the arms-race but the mosquitoes are currently winning (B). This may also suggest that bats highly prefer some other type of prey. The bats may be active throughout the night (C) which seems most likely. Finally, the bats may have a bimodal distribution: a lot of hunting early and late at night with a siesta right around midnight (D). This would suggest that mosquitoes have found their best temporal niche in that dangerous world, i.e, although the bats are not involved in the arms-race, the mosquitoes are and are thus winners, without making the bats "loosers" in the process.
The hypothesis suggests that bats mostly fly around midnight when the mosquitoes are most active, i.e., the bats are winners and mosquitoes loosers in the arms-race (A) . If the peak is at some other point during the night, that would suggest that bats are involved in the arms-race but the mosquitoes are currently winning (B). This may also suggest that bats highly prefer some other type of prey. The bats may be active throughout the night (C) which seems most likely. Finally, the bats may have a bimodal distribution: a lot of hunting early and late at night with a siesta right around midnight (D). This would suggest that mosquitoes have found their best temporal niche in that dangerous world, i.e, although the bats are not involved in the arms-race, the mosquitoes are and are thus winners, without making the bats "loosers" in the process.
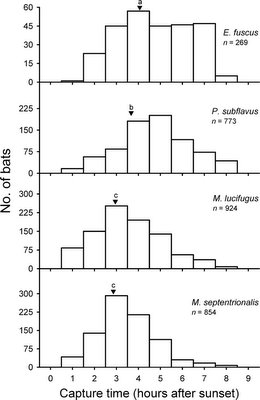
What is the real story? I don't know. Obviously, it is possible to monitor patterns of bat activity [6,7], yet it still needs to be done in regions in which malaria is common. Some of the bats studied in the USA follow predominantly pattern C from the figure above, and it is not too far-fetched to hypothesize that all bats everywhere have similar patterns:
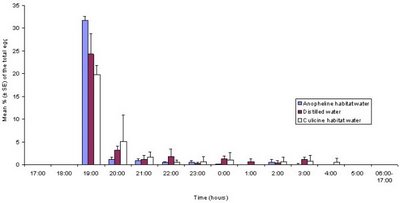
 What are the Anopheles patterns? While they search for blood around midnight, that is not the only time they are flying around. Most of the early part of night is spent looking for mates, mating and laying eggs [8]. Thus, they are easy pickings for bats at times when they are not actively seeking humans. It appears that becoming diurnal is not a good option for Anopheles in the tropics - perhaps there are more birds there than bats, or the birds are more dangerous? It is not impossible for a mosquito to become diurnal - the mosquito we are used to seeing around here - the Culex - is crepuscular (dawn and dusk) and the Asian tiger mosquito is fully diurnal.
What are the Anopheles patterns? While they search for blood around midnight, that is not the only time they are flying around. Most of the early part of night is spent looking for mates, mating and laying eggs [8]. Thus, they are easy pickings for bats at times when they are not actively seeking humans. It appears that becoming diurnal is not a good option for Anopheles in the tropics - perhaps there are more birds there than bats, or the birds are more dangerous? It is not impossible for a mosquito to become diurnal - the mosquito we are used to seeing around here - the Culex - is crepuscular (dawn and dusk) and the Asian tiger mosquito is fully diurnal.
 How does jet-lag figure in here? Apart from the hypothesis stated by Kumar and Sharma that itch sensitivity to mosquito bites gets displaced (and what I added - that temperature rhythm is also displaced), jet-lag will have other effects, too. Let's look at possible effects it may have on people who already have malaria (and you'll see why I had to use so much space describing all of the details of the arms-race above!).
How does jet-lag figure in here? Apart from the hypothesis stated by Kumar and Sharma that itch sensitivity to mosquito bites gets displaced (and what I added - that temperature rhythm is also displaced), jet-lag will have other effects, too. Let's look at possible effects it may have on people who already have malaria (and you'll see why I had to use so much space describing all of the details of the arms-race above!).
Will jet-lag affect the way our body copes with the infection? In a jet-lagged human, there is no clear and sharp rhythm of melatonin release. Some amounts of melatonin are synthetized and secreted at all times of day. This means that the Plasmodium has lost its temporal anchor - there is no signal to use for determination of timing for eruption out of red blood cells. Thus, the gametocytes will errupt at random times - one cell now, another in an hour, another tomorrow. There is no safety in numbers any more - the human immune system is now perfectly capable of dealing with all the plasmodia in the circulation. Of course, the immune system itself may be somewhat compromised in a jet-lagged person.
Will jet-lag affect the way malaria presents its symptoms? The asynchronous eruption of plasmodia also means that there will be no abrupt onset of high fever at midnight. Instead, one may expect a continous low-grade fever. Nightly episodes of high fever are an important symptom of malaria. Will a physician with a patient who exhibits continuous low-grade fever ever suspect malaria? Especially a physician in a country in which there is no malaria and the patient has returned home from the tropical travels and is jet-lagged from a return trip.
Will jet-lag affect the effectiveness of drug treatments? I don't know the details of the way anti-malarial drugs work, so make sure you tell me if I get this all wrong. If the number of plasmodia in the circulation at any time is relatively small, and if the enzymatic destruction of the drug by liver is operating at a constant low rate (instead of with a circadian rhythm of its own), then being jet-lagged should enhance the effectiveness of the drugs, or even allow for the dose to be lowered.
Will jet-lag affect the ability of the patient to be a source of transmittion of the disease to others? With plasmodia erupting at all times of day and with most plasmodia being destroyed by the immune system throughout the day, it is much less likely that any will be present in the skin capillaries at just the right time - at midnight. Also, without a high fever coupled with sweating, the patient is less attractive to the mosquitoes than a malarial patient in the neighboring house who is local and not jet-lagged. Thus, the likelihood of plasmodia being picked up by mosquitoes is even smaller.
To summarize: according to the Kumar/Sharma hypothesis, being jet-lagged increases the chances for contracting malaria. On the other hand, if you already have the disease, it may be good for you to get jet-lagged! As long as you tell your physician that malaria is a serious option so the symptoms are not misinterpreted, you should be better off jet-lagged, allowing your body to fight the disease one plasmodium at a time.
Finally, as a matter of public health policy, how does one get the whole population of malarial patients in one country jet-lagged so as to reduce the transmission rates? Should hospitals induce jet-lag in malaria patients by shifting light-cycles or administering melatonin? How do the pros and cons of such treatment balance? Ah, so many hypotheses, so little data! I hope someone studies this in the future.
One last thing - notice that much of the work described above was performed by researchers outside of USA. Apart from a little bit of cellular physiology, most of the information comes from ecological field-work, and ALL of it is inspired by and informed by evolutionary theory. Not a single gel was run.
Now, I am not dissing molecular biology. Malaria is the only complex parasitic disease in which all players (plasmodium, mosquito and human) have their complete genomes sequenced, and much will be gleaned from such data in terms of designing better anti-malarial drugs, etc. But, as the above research shows, Big (molecular) Biology is not neccessary for findings that have a potential to seriously affect the infection and transmission rates of the disease.
[1] Jet lag and enhanced susceptibility to malaria, C. Jairaj Kumar and Vijay Kumar Sharma, Medical Hypotheses (2006) 66, 671–685
[2] Fooling Anopheles: Scientists Aim to Wipe Out Malaria by Outsmarting a Mosquito's Sense of Smell
[3] Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites, Carlos T. Hotta, Marcos L. Gazarini, Flávio H. Beraldo, Fernando P. Varotti, Cristiane Lopes, Regina P. Markus, Tullio Pozzan and Célia R. S. Garcia, NATURE CELL BIOLOGY , VOL 2, JULY 2000, p.468
[4] Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites, C.T. Hotta, R.P. Markus and C.R.S. Garcia, Braz J Med Biol Res 36(11) 2003
[5] Tertian and Quartan Fevers: Temporal Regulation in Malarial Infection, Célia R. S. Garcia, Regina P. Markus, and Luciana Madeira, JOURNAL OF BIOLOGICAL RHYTHMS, Vol. 16 No. 5, October 2001 436-443
[6] Sampling bats for six or twelve hours in each night? Esberard CEL, Bergallo HG, REVISTA BRASILEIRA DE ZOOLOGIA 22 (4): 1095-1098 DEC 2005
[7] Nightly, seasonal, and yearly patterns of bat activity at night roosts in the Central Appalachians, Agosta SJ, Morton D, Marsh BD, Kuhn KM, JOURNAL OF MAMMALOGY 86 (6): 1210-1219 DEC 2005
[8] Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates, Leunita A Sumba, Kenneth Okoth, Arop L Deng, John Githure, Bart GJ Knols, John C Beier and Ahmed Hassanali, Journal of Circadian Rhythms 2004, 2:6
I have written a little bit about malaria before, e.g, here and here, but this is my special Malaria Action Day post, inspired by a paper [1] that Tara sent me some weeks ago and I never got to write about it till now.
-------------------------------------------------------------------------------
In a journal called "Medical Hypotheses" Kumar and Sharma [1] propose that jet-lagged travellers may be more susceptible to getting infected with malaria. They write:
Rapid travel across several time zones leads to constellation of symptoms popularly known as "jet lag", caused primarily due to mismatch between the timing of circadian clocks of the traveller and the external periodic environment. It is often seen that the jet-lagged individuals who visit their family and friends in areas endemic to malaria have an enhanced susceptibility to malarial infection than the local residents. It would therefore be interesting to explore whether increased susceptibility to malarial infection among the visitors has anything to do with their state of jetlagged.Indeed an interesting hypothesis. Of course, the travellers may also be less resistant to malaria than the locals, or less likely to have a life-style and behavioral patterns conducive to avoiding the mosquito bites, something that may be "second nature" to the locals. They continue:
Individuals with moderate to severe skin response to mosquito bite are largely protected against mosquito borne malaria because itch alerts an individual to mosquito bite and prepares him/her to take necessary precautions to prevent mosquito bite. Itch in an individual follows a diurnal pattern, and it is about hundred folds higher during midnight than midday. A hundred fold increase in itch sensitivity is viewed as a crucial preventive measure against mosquito bites, as this coincides with the midnight flight activity peak of female Anopheles mosquitoes, when she sucks blood from the host after mating peaks in the evening to raise her progeny. Normally individuals residing in endemic areas have their daily peak of itch sensitivity overlapping with the peak – biting phase of female Anopheles mosquitoes. As a result, they are relatively well protected against malarial infection.Interesting idea: if you are sensitive to bites at the time when no mosquitoes are flying and are not sensitive to bites at the time when mosquitoes are flying, you may not get to squash that mosquito in time to prevent the Plasmodia to be injected into your bloodstream. Additionally, a jet-lagged individual may experience a peak of body temperature at night. Mosquitoes, among else, home in on the warmth of their victims. Thus, jet-lagged individuals may be warmer than the surrounding locals at midnight and thus more attractive to mosquitoes at that time.
On the other hand individuals visiting endemic areas from different time zones, particularly during the first couple of days are under the state of jet lag, and their peak protective daily behavioural itch sensitivity lies out of phase with the biting peak of female mosquitoes. Therefore, such individuals are at a greater risk for sustaining malaria compared to the residents. Thus from chronobiological perspective one is of the opinion that a person can be protected against malaria by appropriately adjusting circadian clocks regulating itch sensitivity to the periodic environment. We hope that recent developments in circadian biology will help us predict extent of adjustments necessary in a new environment, which can then be of paramount importance for the protection of jet-lagged travelers to endemic regions against malaria. Some protection against malaria in the chronotherapeutic procedures such as melatonin administration, light therapy, scheduled physical exercise, maximum exposure to new environment during vector free times, social interactions, and appropriate food habits, are a few recommended preventive measures for travelers visiting a malaria endemic areas, in addition to malarial antibiotic prophylaxis.Sounds like good advice, although the administration of melatonin is always an iffy question. However, this hypothesis got my mind all twirling and I came up with some hypotheses of my own. However, it is important to distinguish between different kinds of hypotheses regarding a putative link between jet-lag and malaria. They suggest that jet-lag may:
1) affect the rate/ease of infection with malaria,
2) affect the symptoms of malaria in an infected individual,
3) affect the ability of the body to fight off the infection,
4) affect the effectiveness of treatments, and
5) affect the likelihood that the infected individual will spread the disease to others.
The Kumar/Sharma hypothesis is clearly of the #1 type. I will look more at other types of hypotheses - those that apply to already infected individuals. For that, let's first go quickly through the basic biology of malaria.
 Malaria is caused by a protist in the genus Plasmodium. While Plasmodium falciparum is the most common species, three or four other species are also causes of malaria in humans, and dozens of other species cause malaria or malaria-like diseases in other animals, including mammals, birds and reptiles.
Malaria is caused by a protist in the genus Plasmodium. While Plasmodium falciparum is the most common species, three or four other species are also causes of malaria in humans, and dozens of other species cause malaria or malaria-like diseases in other animals, including mammals, birds and reptiles. Plasmodium is transmitted through bites of several species of mosquito from the genus Anopheles. Once injected into the final host (e.g., human), the plasmodia remain in the skin for several hours, then migrate to lymph nodes, spleen and liver where they undergo several transformations. The final stage - the gametocyte - migrates into the red blood cells. Inside each red blood cell one can find a large number of plasmodia, hiding there from the immune system of the host. The whole life-cycle lasts several days, even weeks to complete.
Plasmodium is transmitted through bites of several species of mosquito from the genus Anopheles. Once injected into the final host (e.g., human), the plasmodia remain in the skin for several hours, then migrate to lymph nodes, spleen and liver where they undergo several transformations. The final stage - the gametocyte - migrates into the red blood cells. Inside each red blood cell one can find a large number of plasmodia, hiding there from the immune system of the host. The whole life-cycle lasts several days, even weeks to complete.
All the plasmodia burst out of red blood cells simultaneously. Enormous number of plasmodia suddenly released into the blood overwhelms the immune system of the host, allowing the plasmodia to survive unscathed for quite a long time. This time is sufficient for them to invade blood vessels in the skin where, if they are lucky, a mosquito will bite and the plasmodia can invade the mosquito again and search for the next host.
 The bursting of red blood cells triggers high fever and sweating. High temperature, high carbon-dioxide, as well as some odors [2] present in the sweat are all highly attractive to mosquitoes, rasing the probability that the host will get bitten. In some species of Plasmodium (like P.falciparum), the bursting of red blood cells occurs every night. In some species of Plasmodium, the resulting fever occurs every two nights and in some every four nights (rarely three), causing, respectively, tertian and quartan fevers. Tertian and quartan malaria are treated by chloroquine, while falciparum malaria is treated by quinine, mefloquine or halofantrine.
The bursting of red blood cells triggers high fever and sweating. High temperature, high carbon-dioxide, as well as some odors [2] present in the sweat are all highly attractive to mosquitoes, rasing the probability that the host will get bitten. In some species of Plasmodium (like P.falciparum), the bursting of red blood cells occurs every night. In some species of Plasmodium, the resulting fever occurs every two nights and in some every four nights (rarely three), causing, respectively, tertian and quartan fevers. Tertian and quartan malaria are treated by chloroquine, while falciparum malaria is treated by quinine, mefloquine or halofantrine.Obviously, from the perspective of a Plasmodium, timing is crucial. First, it is important to errupt in synchrony. Yet, hidden inside red blood cells, plasmodia cannot communicate with each other. Second, it is important to time the eruption in such a way as to maximize the probabilty that some of the gametocytes will be picked up by mosquitoes. Thus, it is important for the eruption to occur at the time of day when mosquitoes are most actively foraging for blood.
How do the Plasmodia solve the problem of timing? This is where circadian biology comes in [3,4,5]. Plasmodia residing inside red blood cells use the time-clues generated by the host. More specifically, they key onto the nightly release of melatonin by the pineal gland. Melatonin is practically undetectable in the blood during the day and the concentrations rise steeply in the evening remaining high for the duration of the night (exact patterns differ between vertebrate species), then dropping again at dawn.
Plasmodia have melatonin receptors [3]. Interestingly, unlike melatonin receptors in vertebrates which are nuclear receptors, the receptors in Plasmodia are membrane receptors. Membrane receptors are much faster than nuclear receptors which is important when a biological event has to be timed with precision. However, the plasmodia do not destroy the red blood cell immediately after receiving the melatonin signal - that would be too early in the evening for the timing to be adaptive, as the mosquitoes are still too busy looking for mates and mating at that time. Instead, the plasmodia use their own circadian clocks to measure the exact timing of eruption. In a way, it appears that the host melatonin signal entrains (synchronizes) the clocks in plasmodia, and then the Plasmodium clock determines the phase (exact timing) for the eruption out of red blood cells.
Different species of Anopheles and even geographically distinct populations of the same species have different times of peak foraging (biting) activity. In each geographical region, the local population (or species) of Plasmodium evolved the timing of eruption to match that of the local mosquitoes.
Let's now introduce another player. Apart from the parasite (Plasmodium), the host (a vertebrate, e.g., a human), and the vector (mosquito), one should also consider the predator - insectivorous bats that hunt for mosquitoes. The way that the malaria literature tends to think about timing can schematically be presented like this:
 There is an assumption that plasmodium eruption, human fever, mosquito foraging and bat hunting are all synchronous. We have already looked at this from the perspective of the Plasmodium - it is adaptive for the Plasmodium for the three bottom lines to be accurate, i.e, that the parasite, the host and the vector are in synchrony. This also means that this is maladaptive to humans. It is also maladaptive to mosquitoes whose fitness does suffer somewhat if they are loaded with parasites.
There is an assumption that plasmodium eruption, human fever, mosquito foraging and bat hunting are all synchronous. We have already looked at this from the perspective of the Plasmodium - it is adaptive for the Plasmodium for the three bottom lines to be accurate, i.e, that the parasite, the host and the vector are in synchrony. This also means that this is maladaptive to humans. It is also maladaptive to mosquitoes whose fitness does suffer somewhat if they are loaded with parasites.On the other hand, it is maladaptive for mosquitoes and plasmodia, and adaptive for humans and bats, if the peak hunting time for bats coincides with the peak foraging time of mosquitoes. More these two events are in sync, more mosquitoes will get eaten, thus less plasmodia will get into a new host and less humans will get infected.
The dynamics of the timing relationship between the four species can be described as an Evolutionary Arms-Race Around The Circadian Clock. While some of the players will try to maximize their fitness by achieving synchrony, the other players maximize their fitness by avoiding synchrony with each other. This can be depicted, for bats and mosquitoes, like this:
 In this case, mosquitoes evolve to forage at later times of night, and bats evolve to track the mosquitoes by hunting later at night. This can go on back and forth endlessly. But, and here is a big "but". This model is quite oversimplified as it posits only four players and for each player an absolute loyalty to the other three. But is the real world that simple?
In this case, mosquitoes evolve to forage at later times of night, and bats evolve to track the mosquitoes by hunting later at night. This can go on back and forth endlessly. But, and here is a big "but". This model is quite oversimplified as it posits only four players and for each player an absolute loyalty to the other three. But is the real world that simple?Plasmodium species are pretty host-specific. Species that thrive inside humans, may not thrive or even survive inside the bodies of other animals and vice versa. So, the parasite is pretty loyal to its host. It is also completely dependent on Anopheles - it will most likely not survive inside a different kind of mosquito.
The same mosquito that usually bites a human will happily take a blood meal from another animal. This is actually used as one of the prevention techniques: a village is surrounded by fields full of cattle, sheep, goats, horses, donkeys or camels. The mosquitoes coming out of the woods at night encounter these animals first and get satiated with blood before they ever encounter humans. The animals themselves do not get sick.
Bats are unlikely, in my opinion, to be specialized on Anopheles as their only prey. If there are no mosquitoes around, they will happily hunt other insects (and the tropical regions where malaria is common are swarming with many species of insects!). I think that involvement of bats in the arms-race is the weakest aspect of the hypothesis. Here are four basic types of bat hunting activity that are theoretically possible:
 The hypothesis suggests that bats mostly fly around midnight when the mosquitoes are most active, i.e., the bats are winners and mosquitoes loosers in the arms-race (A) . If the peak is at some other point during the night, that would suggest that bats are involved in the arms-race but the mosquitoes are currently winning (B). This may also suggest that bats highly prefer some other type of prey. The bats may be active throughout the night (C) which seems most likely. Finally, the bats may have a bimodal distribution: a lot of hunting early and late at night with a siesta right around midnight (D). This would suggest that mosquitoes have found their best temporal niche in that dangerous world, i.e, although the bats are not involved in the arms-race, the mosquitoes are and are thus winners, without making the bats "loosers" in the process.
The hypothesis suggests that bats mostly fly around midnight when the mosquitoes are most active, i.e., the bats are winners and mosquitoes loosers in the arms-race (A) . If the peak is at some other point during the night, that would suggest that bats are involved in the arms-race but the mosquitoes are currently winning (B). This may also suggest that bats highly prefer some other type of prey. The bats may be active throughout the night (C) which seems most likely. Finally, the bats may have a bimodal distribution: a lot of hunting early and late at night with a siesta right around midnight (D). This would suggest that mosquitoes have found their best temporal niche in that dangerous world, i.e, although the bats are not involved in the arms-race, the mosquitoes are and are thus winners, without making the bats "loosers" in the process.What is the real story? I don't know. Obviously, it is possible to monitor patterns of bat activity [6,7], yet it still needs to be done in regions in which malaria is common. Some of the bats studied in the USA follow predominantly pattern C from the figure above, and it is not too far-fetched to hypothesize that all bats everywhere have similar patterns:
 What are the Anopheles patterns? While they search for blood around midnight, that is not the only time they are flying around. Most of the early part of night is spent looking for mates, mating and laying eggs [8]. Thus, they are easy pickings for bats at times when they are not actively seeking humans. It appears that becoming diurnal is not a good option for Anopheles in the tropics - perhaps there are more birds there than bats, or the birds are more dangerous? It is not impossible for a mosquito to become diurnal - the mosquito we are used to seeing around here - the Culex - is crepuscular (dawn and dusk) and the Asian tiger mosquito is fully diurnal.
What are the Anopheles patterns? While they search for blood around midnight, that is not the only time they are flying around. Most of the early part of night is spent looking for mates, mating and laying eggs [8]. Thus, they are easy pickings for bats at times when they are not actively seeking humans. It appears that becoming diurnal is not a good option for Anopheles in the tropics - perhaps there are more birds there than bats, or the birds are more dangerous? It is not impossible for a mosquito to become diurnal - the mosquito we are used to seeing around here - the Culex - is crepuscular (dawn and dusk) and the Asian tiger mosquito is fully diurnal. How does jet-lag figure in here? Apart from the hypothesis stated by Kumar and Sharma that itch sensitivity to mosquito bites gets displaced (and what I added - that temperature rhythm is also displaced), jet-lag will have other effects, too. Let's look at possible effects it may have on people who already have malaria (and you'll see why I had to use so much space describing all of the details of the arms-race above!).
How does jet-lag figure in here? Apart from the hypothesis stated by Kumar and Sharma that itch sensitivity to mosquito bites gets displaced (and what I added - that temperature rhythm is also displaced), jet-lag will have other effects, too. Let's look at possible effects it may have on people who already have malaria (and you'll see why I had to use so much space describing all of the details of the arms-race above!).Will jet-lag affect the way our body copes with the infection? In a jet-lagged human, there is no clear and sharp rhythm of melatonin release. Some amounts of melatonin are synthetized and secreted at all times of day. This means that the Plasmodium has lost its temporal anchor - there is no signal to use for determination of timing for eruption out of red blood cells. Thus, the gametocytes will errupt at random times - one cell now, another in an hour, another tomorrow. There is no safety in numbers any more - the human immune system is now perfectly capable of dealing with all the plasmodia in the circulation. Of course, the immune system itself may be somewhat compromised in a jet-lagged person.
Will jet-lag affect the way malaria presents its symptoms? The asynchronous eruption of plasmodia also means that there will be no abrupt onset of high fever at midnight. Instead, one may expect a continous low-grade fever. Nightly episodes of high fever are an important symptom of malaria. Will a physician with a patient who exhibits continuous low-grade fever ever suspect malaria? Especially a physician in a country in which there is no malaria and the patient has returned home from the tropical travels and is jet-lagged from a return trip.
Will jet-lag affect the effectiveness of drug treatments? I don't know the details of the way anti-malarial drugs work, so make sure you tell me if I get this all wrong. If the number of plasmodia in the circulation at any time is relatively small, and if the enzymatic destruction of the drug by liver is operating at a constant low rate (instead of with a circadian rhythm of its own), then being jet-lagged should enhance the effectiveness of the drugs, or even allow for the dose to be lowered.
Will jet-lag affect the ability of the patient to be a source of transmittion of the disease to others? With plasmodia erupting at all times of day and with most plasmodia being destroyed by the immune system throughout the day, it is much less likely that any will be present in the skin capillaries at just the right time - at midnight. Also, without a high fever coupled with sweating, the patient is less attractive to the mosquitoes than a malarial patient in the neighboring house who is local and not jet-lagged. Thus, the likelihood of plasmodia being picked up by mosquitoes is even smaller.
To summarize: according to the Kumar/Sharma hypothesis, being jet-lagged increases the chances for contracting malaria. On the other hand, if you already have the disease, it may be good for you to get jet-lagged! As long as you tell your physician that malaria is a serious option so the symptoms are not misinterpreted, you should be better off jet-lagged, allowing your body to fight the disease one plasmodium at a time.
Finally, as a matter of public health policy, how does one get the whole population of malarial patients in one country jet-lagged so as to reduce the transmission rates? Should hospitals induce jet-lag in malaria patients by shifting light-cycles or administering melatonin? How do the pros and cons of such treatment balance? Ah, so many hypotheses, so little data! I hope someone studies this in the future.
One last thing - notice that much of the work described above was performed by researchers outside of USA. Apart from a little bit of cellular physiology, most of the information comes from ecological field-work, and ALL of it is inspired by and informed by evolutionary theory. Not a single gel was run.
Now, I am not dissing molecular biology. Malaria is the only complex parasitic disease in which all players (plasmodium, mosquito and human) have their complete genomes sequenced, and much will be gleaned from such data in terms of designing better anti-malarial drugs, etc. But, as the above research shows, Big (molecular) Biology is not neccessary for findings that have a potential to seriously affect the infection and transmission rates of the disease.
[1] Jet lag and enhanced susceptibility to malaria, C. Jairaj Kumar and Vijay Kumar Sharma, Medical Hypotheses (2006) 66, 671–685
[2] Fooling Anopheles: Scientists Aim to Wipe Out Malaria by Outsmarting a Mosquito's Sense of Smell
[3] Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites, Carlos T. Hotta, Marcos L. Gazarini, Flávio H. Beraldo, Fernando P. Varotti, Cristiane Lopes, Regina P. Markus, Tullio Pozzan and Célia R. S. Garcia, NATURE CELL BIOLOGY , VOL 2, JULY 2000, p.468
[4] Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites, C.T. Hotta, R.P. Markus and C.R.S. Garcia, Braz J Med Biol Res 36(11) 2003
[5] Tertian and Quartan Fevers: Temporal Regulation in Malarial Infection, Célia R. S. Garcia, Regina P. Markus, and Luciana Madeira, JOURNAL OF BIOLOGICAL RHYTHMS, Vol. 16 No. 5, October 2001 436-443
[6] Sampling bats for six or twelve hours in each night? Esberard CEL, Bergallo HG, REVISTA BRASILEIRA DE ZOOLOGIA 22 (4): 1095-1098 DEC 2005
[7] Nightly, seasonal, and yearly patterns of bat activity at night roosts in the Central Appalachians, Agosta SJ, Morton D, Marsh BD, Kuhn KM, JOURNAL OF MAMMALOGY 86 (6): 1210-1219 DEC 2005
[8] Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates, Leunita A Sumba, Kenneth Okoth, Arop L Deng, John Githure, Bart GJ Knols, John C Beier and Ahmed Hassanali, Journal of Circadian Rhythms 2004, 2:6


2 Comments:
Wow! What a tour-de-force explanation. I found this fascinating, Bora.
Just some observations, food for thought if you wish, something I am more asking than stating, for commenters to pitch in their explanations.
Post a Comment
<< Home