Let's now continue our series of Clock Tutorials with an introduction to some phenomena (and related terms and concepts) observed in the laboratory in the course of doing standard circadian experiments. Such experiments usually involve either the study of properties of
freerunning rhythms (check the old tutorials, especially
CT2 and
CT 4 for clarification of basic terms and concepts), or the analysis of
entrainment of rhythms to environmental periodicities.
Entrainment is a process by which a biological rhythm is synchronized by an environmental periodicity. In other words, it is the way an external cycle forces the biological clock to assume the
period of the environmental cycle. Imagine that your wristwatch is not very precise and you have to, every day when you wake up in the morning,
reset it to the real local time. This is exactly what the dawn of the day does to myriads of biological clocks residing in gazillions of organisms strewn all over the surface of the Earth (and oceans) every day. It
phase-locks a particular
phase of the circadian rhythm to the particular phase of the day-night cycle (e.g., wake-up time phase to the dawn phase).
Often, it is not immediatelly clear from an
actograph if the apparently entrained rhythm is really entrained. In some cases the environmental cue that is used, e.g., light, exerts a
masking effect. Masking is a direct effect of the environment on the observed/measured output, thus on the hands of the clock as opposed to the core machinery of the clock. For instance, light may directly inhibit locomotor activity in a nocturnal animal (e.g., mouse) or stimulate a rise in body temperature in a diurnal animal (e.g., a bird), regardless to its possible effect on the clock itself.
Thus, it is neccessary to conduct experiments in such a way that a clear distinction between entrainment and masking can be elucidated. This involves, usually, a
freerun-entrainment-freerun protocol, in which the organism is observed in constant conditions for at least a few days, followed by an entrainment regime for at least several days, followed by another bout of monitoring in constant conditions. Visual inspection of the actograph is usually sufficient to discriminate between masking and true entrainment. One first draws a straight line through the onsets of daily activity during the freerun at the top of the actograph. If extending that line through the remainder of the graph places it along the onsets of the second freerun, we conclude that the effect is masking. If the second freerun is "displaced" on the graph compared to the extended line, we are assuming true entrainment. Here is a schematic visual representation of both situations, assuming a diurnal (day-active) endothermic (warm-blooded) animal:

These actographs are
double-plotted for aid in visual inspection of the data. A graph is double-plotted by placing the data of Day 2 both immediately under the Day 1 and immediatelly to the right of Day 1. Day 3 data are also duplicated - on the right of Day 2 in the second row and below Day 2 in the third row, and so on.
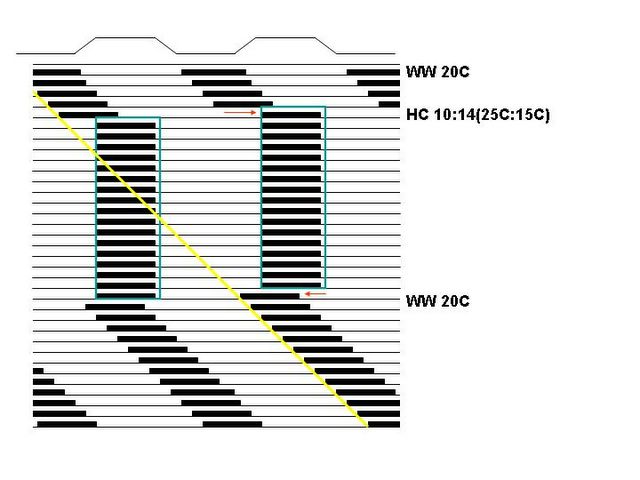
As you can see, a light-dark cycle (as indicated by a white-dark
light bar on top of the graph) entrains the rhythm in the graph on the top, but the temperature cycle (indicated by a line on top of the graph, W=warm, 20 Celsius, H=hot, 25 Celsius, C=cold, 15 Celsius) masks the underlying freerun of the clock in the bottom graph. Thus we conclude that, in this organism, light-dark cycle is an entraining cue (
Zeitgeber), while the temperature cycle is not.
Additional aspects of the records are also helpful. For instance, you may notice that it took a few days of
transients until the rhythm entrained (phase-locked) to the LD cycle. This would not have happened with masking - the shift would have been abrupt. Likewise, at the end of the LD cycle, the freerun starts with the onset-phase (
CT0 - Circadian time) appearing at the time predicted from the onset-phase of the entrained rhythm (
ZT0 - Zetigeber time). In the case of masking, there is often an abrupt (apparent, not real) phase-shift at this point.
Of course, this is just a beginning of your work. Now that you know how your organism responds, you can begin to study the details of physiology of the clock, its entrainment by light, and its non-entrainment by temperature, as well as think about it in evolutionary/adaptive terms.
Not all environmental cycles have such a clear-cut result as the two shown above. It is not necessary that a cycle is either a perfect Zeitgeber or completely ignored by the circadian system. There are shades of grey, too. For instance, some cycles are weak Zeitgebers. Here are some examples of possible results and how they look when plotted as actographs:



The first graph shows a phenomenon called
relative coordination. From the graph you can see that the rhythm is entrained for a few days, then starts drifting (freerunning) away. When it reaches a particular phase relationship with the environmental cycle, it temporarily entrains again, etc. A more abrupt period-change related to this phenomenon is sometimes termed
scalloping (middle graph). If changing phase-relationship between the rhythm and the Zeitgeber results not just in changes of period, but also abrupt changes in phase, this is called
phase-jumping, as seen on the graph on the bottom.
One way of studying entrainment to natural cycles is to force the clocks to entrain to unnatural cycles and chronobiologists have been very creative in inventing strange lighting protocols. I will write about those in more detail once I get to the topic of photoperiodism, but for now, let me introduce you to the two of the simplest types of non-natural cycles that are regularly used in the laboratory, their utility in research, and what kinds of phenomena were noted when these were employed. As light is the strongest and most important Zeitgeber, most of the research employed cycles of light and darkness. However, cycles of other cues (e.g., temperature) have also occasionaly been studied, both in natural and non-natural versions.
Skeleton photocycles contain a normal dark phase, but the dark phase is broken up into three components: the dawn (light), the daytime (dark - lights are off), and dusk (light). Thus a full-photoperiod light-dark cycle composed of 12 hours of light and 12 hours of darkness (LD 12:12), when done as a skeleton photoperiod would look, for instance, like this: 1 hour of light, 10 hours of dark, 1 hour of light, 12 hours of dark (LDLD 1:10:1:12). The duration of light pulses that mimic dawn and dusk is arbitrary, e.g., one can choose to use 1 second, or 3 hours, depending on the hypothesis one is testing. If the dawn-pulse and the dusk-pulse are of the same duration (e.g., each is 2 hours long), this is called a
symmetrical skeleton photoperiod. If one is longer than the other (usually dawn longer than dusk, e.g., a 6-hour dawn pulse and a 1-hour dusk pulse), this is an
assymetrical skeleton photoperiod.
If the stimulus used is just having a masking effect, there will be a response to the two pulses, but no response seen during the darkness in-between the pulses (bottom plot). On the other hand, if the skeleton photocycle results in true entrainment, the observed overt rhythm will look the same whether it is exposed to the full or to the skeleton cycle (top and middle plots):

One interesting observation from the studies of entrainment by skeleton photoperiods is the
bistability phenomenon. A full photoperiod will entrain the biological clock no matter how short or long is the
photoperiod, i.e., the duration of the light portion of the light-dark cycle. Thus LD 1:23, LD 3:21, LD 6:18, LD 8:16, LD 10:14, LD 12:12, LD 14:10, LD 16:8, LD 18:6, LD 21:3 and LD 23:1 are all equally likely to entrain a clock. On the other hand, with skeleton photocycles, photoperiod matters. The biological clock "prefers" short photoperiods to long photoperiods. Thus, when exposed to a skeleton photocycle that attempts to entrain to a cycle mimicking a long photoperiod, the rhythm will, instead, reverse the "night" and "day" (often with an abrupt phase-jump). For instance LD 1:16:1:6 will not be understood by the clock as LD 18:6, but as LD 6:18.
In nature, many nocturnal (night-active) organisms actually live in skeleton photoperiods. During the night, they are out foraging. During the day, they are hiding (and sleeping) deep in the darkness of their burrows or caves. The only light they see are very brief periods during dawn and dusk. Thus, this type of lighting protocol in the laboratory has an additional utility in that it studies a natural phenomenon.
On the other hand, outside of science-fiction, there is nothing natural about
T-cycles. With these, the total duration of the cycle (e.g,.
L + D = T ) is not equal to 24 hours. Usually groups of organisms are exposed to a systematic array of T-cycles, e.g., LD 6:6, LD 6:9, LD 6:12, LD 6:15, LD 6:18, LD 6:21, LD 6:24, LD 6:27, LD 6:30, etc. The first four are shorter than 24 hours, the fifth is exactly 24 hours, and the last four are longer than 24 hours. If an organism is exposed to a T-cycle to which it cannot entrain, it may, instead, exhibit a phenomenon called
frequency demultiplication. For instance, it may entrain to LD 6:6 as if it was an LD 18:6 (or a skeleton cycle LDLD 6:6:6:6). In other cases, the rhythm may show relative coordination, or just ignore the cycle altogether and freerun through the whole experiment.
In some cases, for the ease of visual inspection, the actograph is not
folded at 24 h, but at the period of the cycle. For instance, on the top is a schematic of entrainment to a T-cycle folded at 24 hours, and on the bottom is the same record folded at 22 hours:

This type of protocol uncovers the
limits of entrainment for each species. Most rodents used in laboratory research have very narrow limits of entrainment, with T (period of the cycle) ranging somewhere between 23h and 25h. On the other hand, plants, fungi and protists often have very large limits of entrainment. Of course, there is quite a lot of variation between related groups, thus sparrows have much narrower limits of entrainment than quail, although both are birds. Adaptive function of limits of entrainment has over the years been the subject of some study, but no clear conclusions can be made yet. The differences between phyla are particularly difficult to understand, while differences within smaller group sometimes correrelate with territory-size, burrowing vs. surface nesting, diurnality vs. nocturnality, or sedentary vs. migratory life history. For instance, the limits can change within the same animal, depending if it is in its migratory condition or not. Much more work needs to be done before anything concrete can be said about the physiology, the evolution and the adaptive function of limits of entrainment.
Conditions of entrainment may have either transient or lasting effects on the subsequent freerunning period in constant conditions. Such effects are called
aftereffects. Exposure to different photoperiods may induce aftereffects, for instance exposure to a short photoperiod may result in a shorter subsequent period, and exposure to the longer photoperiod in a longer subsequent freerunning period. However, the largest aftereffects are seen after exposure to T-cycles: usually short T-cycles causing subsequent shorter periods, and longer T-cycles causing longer freerunning periods.
This phenomenon, although its cause and mechanism are still elusive, was used in some creative experiments. Exposure of two groups of animals to two different T-cycles results in a creation of two colonies of animals with different endogenous periods. Transplantation of candidate pacemaker tissues between the two groups than reveals if the tissue is actually a pacemaker. If the animal's rhythms continue with the period of the host, the transplant is not a pacemaker. If the host animal assumes the period of the donor, the tissue is a pacemaker. This protocol is especially valuable in species in which there are no known naturally occuring period mutations and are not amenable to genetic engineering. Terry Page used this protocol to uncover the pacemaking tissues in the cockroach. More recently, he used aftereffects (of entrainment to T-cycles) in another creative experiment. He exposed two groups of cockroaches to inhibitors of transcription and translation for several hours. Result: aftereffects were retained. The expectation was that such a treatment would transiently stop the clock, which would then re-start with its own, genetically determined "natural" freerunning period. However, the blocked cells managed, somehow, to "remember" the aftereffects through the duration of the treatment, suggesting that the clock-gene transcription-translation feedback loop is not necessary for determination of the most basic property of the circadian rhythm - its period.
I will return to T-cycles and skeleton photoperiods later, when I discuss photoperiodic phenomena. Let me now turn to a different topic: the
parametric and
non-parametric effects of light. Parametric effects of light are effects of intensity and wavelength of light on the rhythm. Non-parametric effects of light are efects of timing of onsets and offsets of light-pulses (or longer pulses, e.g., photoperiods).
Exposure to constant bright light will often result in arrhythmicity (at the level of the organism - see
this post for evidence that individual pacemaker cells keep cycling in mutual asynchrony). In plants, exposure to constant darkness may result in arrhythmicity, mainly due to the plant's photosynthetic needs.
In nocturnal animals, brighter the constant light, longer the period (
tauLL > tau DD). In diurnal animals, the reverse is true (
tauLL less than tau DD), though there are many more examples of diurnal than nocturnal animals disobeying this rule (either behaving as nocturnal animals, or ignoring the parametric effects of light entirely). Since a relatively recent ancestor of all mammals was a nocturnal burrowing insectivore-like creature, it is likely that some mammals evolved diurnality comparatively recently and retained a nocturnal response to light intensity (I tried to post the image five times and it just won't "take" - I managed to post it in the Archives, though, so click on this to see it:

This whole phenomenon, often written in shorthand with the two little formulas I wrote in the parentheses, was first discovered by Jurgen Aschoff. In 1960., Colin Pittendrigh suggested that this be called
Aschoff's Rule, which everyone in the field accepted. The most prestigious award in chronobiology is called, appropriately, Aschoff's Ruler, an actual classroom ruler with names of past winners inscribed on it. The way the award is given is unusual. There is no nominating committee involved. The recipient of Aschoff's Ruler chooses the next year's recipient. But, the two consecutive winners cannot live on the same continent, nor work on the same model organism, or have ever worked on the same organism in their whole scientific careers, which made it difficult for some people who have studied many species, e.g., Mike Menaker, but he got it somehow (he works on a variety of vertebrates, the previous winner, from Europe, works on fungi and protists, and the subsequent winner, from Japan, was using only rats).
In some organisms, exposure to constant bright light results in a phenomenon called
splitting. Two (or more) components of the circadian rhythm start freerunning each with its own endogenous period. Thus, when one inspects the actograph record, the components diverge from each other, cross over each other, or fuse again, and this phenomenon usually repeats over and over again. The observation of splitting in the tree-shrew (
Tupaia), and subsequently in some other organisms, was an early suggestion (in the history of the field) that organisms possess more than one clock and that multiple clocks may be organized in more complex circadian systems (see
CT 5: Circadian Organization).
Study of parametric effects of light continues, with emphasis on effects of gradual dawn and dusk (as opposed to usual laboratory practice of switching the lights on and off abruptly), as well as the roles of various photopigments (e.g.,
melanopsin, cryptochrome, rhodopsin, pinopsin etc.) and photosensitive tissues (retina, pineal, deep-brain photoreceptors) in detection of different components of natural light, e.g., its wavelength and intensity, both of which change over the course of the day.
Study of non-parametric effects of light analyzes effects of
light-pulses (or photoperiods) on phase of the freerunning rhythm. Onsets and offsets of light pulses usually result in
phase-shifting of the rhythm. Analysis of such
phase-shifts can teach us about the mechanisms of entrainment to full daily exposures to light and darkness. For instance, the difference between abrupt shifts (the phase-shift is completed in one cycle) and
transients (rhythm assumes a new phase after several days of gradual shifting) can be very informative about the underlying physiology.

Non-parametric effects of light, as postulated by Colin Pittendrigh, have been studied more extensively and are much better understood. Parametric effects of light, as proposed by Jurgen Aschoff, are less well understood. The only person who studied and published with both of them, Serge Daan, has attempted in recent years to combine the two approaches. In the next couple of weeks, I intend to write a series of posts about
formal analysis of non-parametric effects of light (constructing the
Phase-Response Curve), as well as attempts to put together parametric and non-parametric effects (e.g., transient-response curve, tau-response curve, application of
limit cycles) and, in addition, the utility of such approaches to the study of biological rhythms. This kind of stuff is pretty hard to grasp and contains some heavy-duty math, so I will try to go slow and make it as simple as possible. Still, you are all probably tired of all this theory by now, so I will take a little detour in the meantime and write the next couple of posts on some real anatomy and physiology of circadian systems in mammals and non-mammalian vertebrates before I come back to the theoretical aspects of chronobiology.
Archives/Categories:
Clock TutorialsTags:
circadian























