Understanding the role of serotonin in depression has led to development of anti-depressant drugs, like Prozac. Much of the
research in this area has been performed in Crustaceans: lobsters and crayfish. The opposite behavioral state of depression, something considered a normal state, could possibly best be described as self-confidence.
Self –confidence is expressed differently in different species, but seems to always be tied to high status in a social hierarchy. In crayfish, self-confidence is expressed as aggression. When two individuals meet, they engage in aggressive behavior (Fig. 1, see below).
Outcome of this “fight” determines the social status and leads to long-term changes in the brain, particularly the serotonergic system, associated with either winning (self-confidence) or losing (depression). Thus, experiencing a victory alters the behavior in a way that makes subsequent victories more likely and vice versa (Goessmann et al., 1999; Huber et al., 1997; Issa et al., 1999). Experimental manipulations of the serotonergic system by, for instance, injections of serotonin or Prozac, result in changes in aggressive behavior and social status.
Two standard experimental practices are used in the study of aggression in crustaceans. In one, two or more individuals are placed together in an aquarium and left there for a long period of time (days to weeks). After the initial aggressive encounters, the social status of an individual can be deduced from its control of resources, like food, shelter and mates.
In the other paradigm, two individuals are allowed to fight for a brief period of time (less than an hour), after which they are isolated again and re-tested the next day at the same time of day. Neither of the two approaches is capable of addressing two questions: how fast do the changes in the brain start to occur, and, what if any effect can time of day have on the behavior. Because of this, the existing literature, sometimes implicitly sometimes explicitly, suggests that the rule "once a winner, always a winner" is true, and that the neural changes are fast and induced by a single agonistic encounter.
To address these two questions, Amy Hughes, then an undergraduate student in our department, now an epidemiology graduate student at the University of Minnesota, and I have designed a different experimental procedure. This study began as a project in the Animal Anatomy and Physiology laboratory class in which Amy was my student. However, the initial data were interesting and we easily persuaded Dr.Robert Grossfeld to help support further study as Amy's honors research project. Apart from securing funding, animals, equipment and space, Dr.Grossfeld proved as a valuable resource on crustacean neurobiology and on keeping the research focused and 'on target'.
We kept individual crayfish in social isolation for a month prior to the experiment. They were kept in a light-dark cycle consisting of 12 hours of light and 12 hours of darkness. Then, we matched the individuals by size and tested their aggressive behavior and social status by behavioral observations for about 15 minutes every 3 hours over a period of 24 hours. One set of tests was initiated at “dawn” or lights-on time, which was 8am and ended at 8am on the next day. The other set of tests started at dusk, or lights-off time, which was 8pm and ended at 8pm the following day. We scored the behaviors using the scoring systems modified from standard literature (Karavanich and Attema 1998, see Table 1).
It has been known for quite a long time now that crayfish are nocturnal animals and that the levels of general locomotor activity are much greater during the night than during the day (Page and Larimer 1972). However, no specific behaviors were monitored in such studies. Analysis of our data shows that aggressive behavior is much more intense and frequent during first three hours of night than at other times during the 24-hour cycle (Fig.2). This is somewhat different from the general locomotor activity which tends to be high throughout the night, not concentrated to the early portion of the night.
Expectation that brain changes associated with winning or losing are initiated very fast were met by about half of the tested pairs of animals in each data-set. In these animals, the social status was determined during the first or second time-point and remained stable for the rest of the experiment (Figs 3 and 4).
However, in about a half of the pairs in each data-set, the stable social hierarchy was not achieved during the 24-hour period of the experiment. Levels of aggression and the final outcome varied during this period, suggesting that the brain changes in these individuals did not occur as fast, and that there is considerable plasticity in behavioral states during the first 24 hours of social interactions (Fig. 5).
Interestingly, more switches in social status occurred during the day than during the night. This can be explained in several different ways. For instance, a very high level of aggression may be necessary to trigger the changes in the brain, and such highly aggressive encounters are most likely to occur in the early night. Thus, encounters during the previous day were not intense enough to result in long-term changes in the brain. Further research needs to be done to study the rate at which an experience of victory or loss triggers permanent changes in the brain.

Also, there is no strong evidence in the literature that the two individuals can actually recognize each other. However, they can recognize the social status of their opponents. During the fights, crayfish squirt urine at each other (Zulandt Schneider et al. 2001, also see figure on the right where urine is colored with a fluorescent dye). Chemical signature of the urine was not studied at all so far, but it apparently carries information about the brain-state of the animal.
Since socially dominant animals have higher levels of serotonin in their nervous system, it is likely that the chemical message in the urine is related to serotonin. The most likely candidate, a direct metabolite of serotonin, is melatonin. A recent study has shown that levels of melatonin in these animals are very high in the early night, drop off somewhat during the rest of the night and are practically undetectable during the day (Tilden et al. 2003). Our results are consistent with the notion that urinary melatonin (or its metabolite) may be the signal carrying information about social status, as the most intense fights and most definitive outcomes occurred during the early night. This hypothesis needs to be tested in the future.
This research has been displayed on the poster session of the Undergraduate Research Sympsium at North Carolina State University. It is unlikely that any of the participants in this study will follow up on this work in the near future. Though there is not sufficient data to publish a 'real' paper, we feel that the information should be available online for those who study aggression, social dominance, serotonin, melatonin and circadian rhythms in crustaceans.
The project, as presented by Amy on the poster, is reproduced below. We will appreciate
proper attribution in case you follow up on this study. The correct reference is:
Amy Hughes, Bora Zivkovic and Robert Grossfeld, Influence of Light Cycle on Dominance Status and Aggression in Crayfish (April 6, 2006), Circadiana blog; http://circadiana.blogspot.com/2006/04/influence-of-light-cycle-on-dominance.html
Influence of Light Cycle on Dominance Status and Aggression in Crayfish
Amy Hughes, Bora Zivkovic and Robert Grossfeld
INTRODUCTIONConflicts arise within a population of social animals over the allocation of important resources. Interactions between crayfish determine a social hierarchy both when resources are in dispute and when they are not. Even when no resource except space is contested, crayfish will fight. Several factors determine the outcomes of these contests, including physical attributes of an organism and past experience (Goessmann et al., 1999; Huber et al., 1997; Issa et al., 1999; Zulandt Schneider et al., 2000).
In most previous studies of crustacean aggressive behavior, animals were tested at the same time for consecutive days. In those experiments, winning enhanced further success by heightening aggression, whereas losing decreased subsequent chances for dominance by lowering aggression.
Recognition of the opponent’s attributes plays a role in changes in social status, e.g. detection of the opponent’s relative dominance status and recognition of a prior opponent (Goessmann et al., 1999; Karavanich & Attema, 1998; Zulandt Schneider et al., 2000).
The purpose of this study was to test the hypothesis that the establishment and stability of a dominance hierarchy may be plastic over sequential pairings during the first day, and influenced by time of day.
MATERIALS AND METHODS- Forty-four adult male crayfish (Procambarus clarkii) were kept in separate containers with no visual or physical contact for one month on a LD12:12 light cycle (lights on automatically at 8:00 AM and lights off at 8:00 PM). They were fed fish food pellets every 7 days.
- Pairs of 20-40 g inter-molt males were matched closely by weight (within 1 g).
- For testing, each pair was placed in a small container [30 cm (L) x 20 cm (W) x 15 cm (D)] filled approximately two-thirds full with fresh water.
- The same pairs encountered each other for approximately 15 min every 3 h over a 24-h period.
- One set (11 pairs) was tested first at the time of lights-off (i.e. 8:00 PM).
- The other set (11 pairs) was tested first at the time of lights-on (i.e. 8:00 AM).
- The time of initiation of a fight, duration of the fight, number and type of agonistic behaviors, and final “winner” of the encounter were recorded based on visual observation. The total agonistic behavior scores were compared using a one-tailed Student t- test.
Figure 1. Two crayfish in a fight
Table 1. The scoring table of behavior. Modified from Karavanich and Attema 1998. Insert: an example of a meral spread.
Retreat – moving away, turning away: -1
Strike and Rip – using claws unrestrained: +4
Claw Lock – using claws to grasp: +3
Meral Spread – threatening display of claws: +2
Approach – walking towards, turning towards other animal: +1
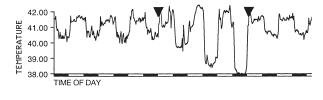
Figure 2: Agonistic interactions escalate during the early evening following lights off (i.e. 8:00 PM). n=44; p<0.0001. Figure 3: Behavior scores of crayfish tested first at 8:00 PM
Figure 3: Behavior scores of crayfish tested first at 8:00 PMIn 7 of 11 pairs, the animal that established dominance at the first or second time point (8:00 PM or 11:00 PM, respectively) remained the more aggressive animal the entire 24 h.
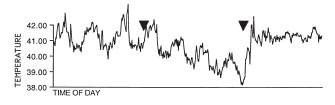
 Figure 4: Behavior scores of crayfish tested first at 8:00 AM
Figure 4: Behavior scores of crayfish tested first at 8:00 AMIn 5 of 11 pairs, the animal that established dominance at the first or second time point (8:00 AM or 11:00 AM, respectively) remained the more aggressive animal the entire 24 h.
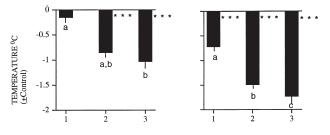
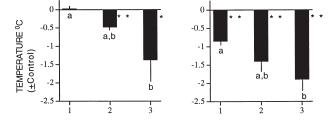
 Figure 5: In 10 pairs, a stable dominance relationship was not established during the first two time points.
Figure 5: In 10 pairs, a stable dominance relationship was not established during the first two time points.The number of switches in social status was greater during the light phase than during the dark phase (p<0.01). In 4 of 11 pairs (8:00 PM set), dominance switched over the 24 hours:

In 6 of 11 pairs (8:00 AM set), dominance switched over the 24 hours:
 CONCLUSIONS
CONCLUSIONSCrayfish agonistic behaviors are most frequent during early evening following lights-off as compared to early morning following lights-on.
In about half of the pairs, the relative levels of aggression (“dominance status”) remained stable after the first or second encounter, whereas in the other pairs it changed at one or more times during the 24 hour testing period, especially during the daytime. The latter results suggest plasticity in social status during the first day that crayfish are paired.
REFERENCESBalzer I.; Espinola I.R.; Fuentes-Pardo B., Daily variations of immunoreactive melatonin in the visual system of crayfish, Biology of the Cell, Volume 89, Number 8, November 1997, pp. 539-543(5)
Goessmann, C., Hemelrijk, C., and Huber, R. (2000). The formation and maintenance of crayfish hierarchies: Behavioral and self-structuring properties. Behav Ecol Sociobiol. 48:418-428.
Huber, R., Smith, K., Delago, A., Isaksson, K., and Kravitz, E. A. (1997). Serotonin and aggressive motivation in crustaceans: Altering the decision to retreat. Proc. Natl. Acad. Sci. USA. 94: 5939-5942.
Issa, F.A., Adamson, D.J. and Edwards, D.H. (1999). Dominance hierarchy formation in juvenile crayfish
Procambarus clarkii. J. Exp. Biol. 202:3497-3506.
Karavanich, C. and Attema, J. (1998). Individual recognition and memory in lobster dominance. An. Behav.. 56:1553-1560.
Page,Terry L. and Larimer, James L. (1972), Entrainment of the circadian locomotor activity rhythm in crayfish: The role of the eyes and caudal photoreceptor, Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, Volume 78, Number 2: 107 - 120
Tilden, Andrea R. , Brauch Rebecca, Ball Ryan, Janze Aura M. ,Ghaffari Ali H. , Sweeney Catherine T., Yurek Jamie C. and Cooper Robin L. (2003), Modulatory effects of melatonin on behavior, hemolymph metabolites, and neurotransmitter release in crayfish, Brain Research 992: 252–262
Zulandt Schneider, R. A., Huber, R. and Moore, P.A. (2001), Individual and status recognition in the crayfish,
Orconectes rusticus: The effects of urine release on fight dynamics. Behav. 138:137-153.

 In the first study (click on images to enlarge), hydrogenase uptake was measured in unoxic (anaerobic) conditions in constant light (LL) at 32oC, and in constant darkness at 32oC and 16oC. In each of the three conditions, a rhythm was observed. The period of the freerunning rhythms was markedly different between the three conditions. In LL-32oC, period was ultradian: 12.1 hours. In DD at 32oC, the period was also ultradian: 14.8 hours. Only in DD at 16oC was the rhythm within a circadian range: 23.4 hours.
In the first study (click on images to enlarge), hydrogenase uptake was measured in unoxic (anaerobic) conditions in constant light (LL) at 32oC, and in constant darkness at 32oC and 16oC. In each of the three conditions, a rhythm was observed. The period of the freerunning rhythms was markedly different between the three conditions. In LL-32oC, period was ultradian: 12.1 hours. In DD at 32oC, the period was also ultradian: 14.8 hours. Only in DD at 16oC was the rhythm within a circadian range: 23.4 hours.
 In the second study, light output was measured in three experiments. In all three, bacteria were assayed in constant darkness at 23oC. In the first and second groups, bacteria were pre-treated and their putative clocks entrained by a warm-cold-warm cycle prior to release into constant conditions. In the third group, the pre-treatments was exposure to a light-dark cycle prior to release into constant conditions. The first group was tested under aerobic conditions, while the second and the thir group were tested under anaerobic conditions.
In the second study, light output was measured in three experiments. In all three, bacteria were assayed in constant darkness at 23oC. In the first and second groups, bacteria were pre-treated and their putative clocks entrained by a warm-cold-warm cycle prior to release into constant conditions. In the third group, the pre-treatments was exposure to a light-dark cycle prior to release into constant conditions. The first group was tested under aerobic conditions, while the second and the thir group were tested under anaerobic conditions.